

Use the diagram below to identify the products you need in this compatibility system. Underneath the diagram you will find the listing of all the products for this diagaram where you can add it to the quote basket and request a quote.
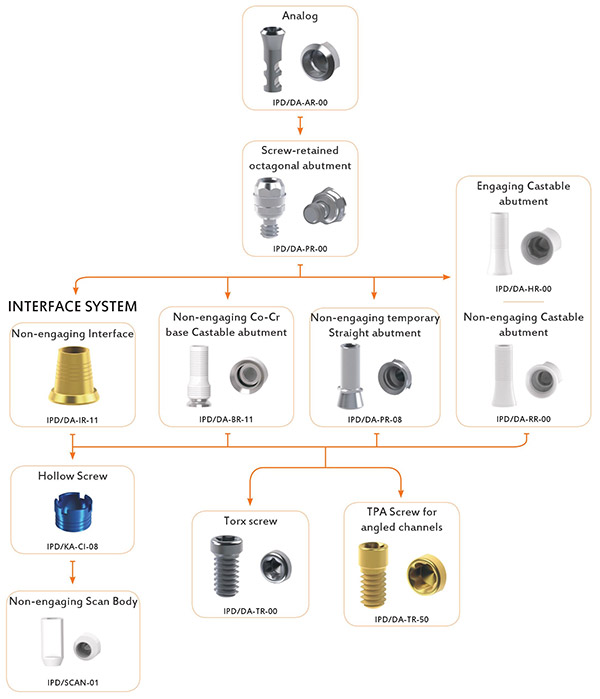

Article Code: IPD/DA-AR-00
Description: Implant replica for use in the laboratory
Reusable: No
Material: AISI 303: Stainless material with excellent mechanical properties.

Article Code: IPD/DA-PR-00
Description: Cementing abutment Engaging Ø 4,8
Reusable: No
Material: Titanium Grade 5

Article Code: IPD/DA-HR-00
Description: Castable Engaging synOcta Ø 4,8
Reusable: No
Material: POM C

Article Code: IPD/DA-RR-00
Description: Castable Non-Engaging synOcta
Ø 4.8
Reusable: No
Material: POM C

Article Code: IPD/KA-CI-08
Description: Ti Screw
Torque:10 Ncm
Reusable: No
Material: Titanium Grade 5

Article Code: IPD/SCAN-01
Description: Non-Engaging Hollow Interface Scan Abutment
Reusable: No
Material: Peek

Article Code: IPD/DA-TR-00
Description: Ti Screw synOcta Ø 4.8
Torque: 15 Ncm
Screw: Torx
Reusable: No
Material: Titanium Grade 5

Article Code: IPD/DA-TR-50
Description: TPA Screw Ø 4.8
Torque: 20 Ncm
Key: Angulados
Reusable: No
Material: Titanium Grade 5